Topic: Hydrocarbons, Test No.: 01, Total MCQs: 15
Congratulations - you have completed Topic: Hydrocarbons, Test No.: 01, Total MCQs: 15.
You scored %%SCORE%% out of %%TOTAL%%.
Your performance has been rated as %%RATING%%
Question 1 |
Toluene | |
Benzene | |
Benzoic Acid | |
Nitrobenzene |
Question 2 |
Tertiary butyl chloride | |
Neopentane | |
Isohexane | |
Neohexane |
Question 3 |
Clemmensen reduction | |
Wurtz reaction | |
Wurtz-Fittig reaction | |
Friedel-Crafts reaction |
Question 4 |
Identify B and D is the following sequence of reactions. [Karnataka CET 2011]
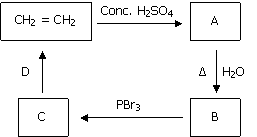
Ethanol and alcoholic KOH | |
Methanol and bromoethane | |
Ethyl hydrogen sulphate and alcoholic KOH | |
Ethyl hydrogen sulphate and aqueous KOH |
Question 5 |
1 - Pentene | |
2 - Methyl - 1 - pentene | |
2 - Methyl - 2 - pentene | |
2 - Pentene |
Question 6 |
A vinyl group | |
Two ethylenic double bonds | |
An acetylenic triple bond | |
An isopropyl group |
Question 7 |
The non-aromatic compound among the following is [AIEEE 2011]
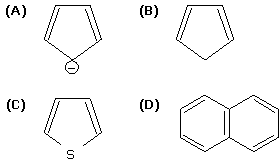
Option (A) | |
Option (B) | |
Option (C) | |
Option (D) |
Question 8 |
2, 2-dibromobutane | |
1, 1-dibromopropane | |
1, 4-dibromobutane | |
1, 2-dibromoethane |
Question 9 |
Heat of hydrogenation of benzene is less than the theoretical value | |
There are three carbon-carbon single bonds and three carbon-carbon double bonds | |
It forms only one type of monosubstituted product | |
The bond angle between carbon-carbon bonds is 120° |
Question 10 |
The synthesis of 3-octyne is achieved by adding a bromoalkane into a mixture of sodium amide and an alkyne. The bromoalkane and alkyne respectively are [IIT JEE 2010]
BrCH2CH2CH2CH2CH3 and CH3CH2C ≡ CH | |
BrCH2CH2CH3 and CH3CH2CH2C ≡ CH | |
BrCH2CH2CH2CH2CH3 and CH3C ≡ CH | |
BrCH2CH2CH2CH3 and CH3CH2C ≡ CH |
Question 11 |
Ethane | |
Propene | |
1-butene | |
2-butene |
Question 12 |
propyne | |
propene | |
propane | |
propanol |
Question 13 |
The angle strain in cyclobutane is [Karnataka CET 2008]
24°44′ | |
29°16′ | |
19°22′ | |
9°44′ |
Question 14 |
The overlapping of orbitals in benzene is of the type [Karnataka CET 2008]
sp – sp | |
p – p | |
sp2 – sp2 | |
sp3 – sp3 |
Question 15 |
In the following sequence of reactions, the alkene affords the compound 'B'
CH3
CH = CHCH3 ![]()
The compound B is [AIEEE 2008]
CH3CH2CHO | |
CH3COCH3 | |
CH3CH2COCH3 | |
CH3CHO |

