JEE MAIN, AIPMT, Chemistry Question Papers - MCQs Test 1
Quiz-summary
0 of 15 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
Information
JEE MAIN, AIPMT, Chemistry Question Papers – MCQs Test 1
The Online Test consists of 15 questions. Each question is allotted 4 (four) marks for each correct response. The maximum marks are 60. The test is of 30 minutes duration. After you attempt the test, click “Quiz-summary” at the end.
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading...
You must sign in or sign up to start the quiz.
You have to finish following quiz, to start this quiz:
Results
0 of 15 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 points, (0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- Answered
- Review
-
Question 1 of 15
1. Question
4 points+I effect is shown by
Correct
Incorrect
-
Question 2 of 15
2. Question
4 points0.023 g of sodium metal is reacted with 100 cm3 of water. The pH of the resulting solution is ______.
Correct
Incorrect
-
Question 3 of 15
3. Question
4 points0.5 mole of each of H2, SO2 and CH4 are kept in a container. A hole was made in the container. After 3 hours, the order of partial pressures in the container will be
Correct
Incorrect
-
Question 4 of 15
4. Question
4 points10 cm3 of 0.1 N monobasic acid requires 15 cm3 of sodium hydroxide solution whose normality is
Correct
Incorrect
-
Question 5 of 15
5. Question
4 points10-6 M NaOH is diluted 100 times. The pH of the diluted base is
Correct
Incorrect
-
Question 6 of 15
6. Question
4 points2 gm of a radioactive sample having half life of 15 days was synthesised on 1st Jan 2009. The amount of the sample left behind on 1st March. 2009 (including both the days)
Correct
Incorrect
-
Question 7 of 15
7. Question
4 points2HI(g) Ý H2(g) + I2(g)
The equilibrium constant of the above reaction is 6.4 at 300 K. If 0.25 mole each of H2 and
I2 are added to the system, the equilibrium constant will be
Correct
Incorrect
-
Question 8 of 15
8. Question
4 points2SO2(g) + O2(g)
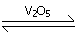 is an example forCorrect
is an example forCorrect
Incorrect
-
Question 9 of 15
9. Question
4 points30 cc of
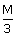 HCl, 20 cc of
HCl, 20 cc of 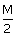 5 HNO3 and 40 cc of
5 HNO3 and 40 cc of 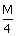 NaOH solutions are mixed and the volume was made up to 1 dm3. The pH of the resulting solution isCorrect
NaOH solutions are mixed and the volume was made up to 1 dm3. The pH of the resulting solution isCorrect
Incorrect
-
Question 10 of 15
10. Question
4 points5 moles of SO2 and 5 moles of O2 are allowed to react. At equilibrium, it was found that 60% of SO2 is used up. lf the partial pressure of the equilibrium mixture is one atmosphere. the partial pressure of O2 is
Correct
Incorrect
-
Question 11 of 15
11. Question
4 pointsWhich one of these is NOT TRUE for benzene?
Correct
Incorrect
-
Question 12 of 15
12. Question
4 pointsWhich one of the following is paramagnetic?
Correct
Incorrect
-
Question 13 of 15
13. Question
4 pointsWhich one of the following is a covalent crystal?
Correct
Incorrect
-
Question 14 of 15
14. Question
4 pointsWhich one of the following DOES NOT involve coagulation?
Correct
Incorrect
-
Question 15 of 15
15. Question
4 pointsWhich one is not a constituent of nucleic acid?
Correct
Incorrect


please provide answers and explanations