Sr. No. |
Question |
Answer |
| 1. |
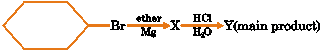
Given reaction
Y in the reaction is (A) Hexane (B) Cyclohexane (C) Cyclohexylcyclohexane (D) Cyclohexylether |
Answer: (B) |
| 2. |
Given the following reactions involving A, B, C and D (i) C + B+ → C+ + B (ii) A+ + D → No reaction (iii) C+ + A → No reaction (iv) D + B+ → D+ + B The correct arrangement of A, B, C, D in order of their decreasing ability as reducing agent (A) D > B > C > A (B) A > C > D > B (C) C > A > B > D (D) C > A > D > B |
Answer: (D) |
| 3. | Glucose contains in addition to aldehyde group
(A) one secondary OH and four primary OH groups (B) one primary OH and four secondary OH groups (C) two primary OH and three secondary OH groups (D) three primary OH and- two secondary OH groups |
Answer: (B) |
| 4. | H2S is passed into one dm3 of a solution containing 0.1 mole of Zn2+and 0.01 mole of Cu2+ till the sulphide ion concentration reaches 8.1 × 10–19 moles. Which one of the following statements is true? [Ksp of ZnS and CuS are 3 × 10–22 and 8 × 10–36 respectively]
(A) Both CuS and ZnS precipitate (B) Only ZnS precipitates (C) No precipitation occurs (D) Only CuS precipitates |
Answer: (A) |
| 5. | Hybridization of C2 and C3 of H3C – CH = C = CH – CH3 are
(A) Sp, Sp3 (B) Sp2, Sp (C) Sp2, Sp2 (D) Sp, Sp |
Answer: (B) |
| 6. | Hydrogen bond is strongest in
(A) S — H —— O (B) O — H —— S (C) F — H —— F (D) O — H —— N |
Answer: (C) |
| 7. | Hydrogen is prepared from H2O by adding
(A) Ca, which acts as reducing agent (B) AI, which acts as oxidising agent (C) Ag, which acts as reducing agent (D) Au, which acts as oxidising agent |
Answer: (A) |
| 8. | Identify B and D is the following sequence of reactions.
(A) Ethyl hydrogen sulphate and alcoholic KOH (B) Methanol and bromoethane (C) Ethanol and alcoholic KOH (D) Ethyl hydrogen sulphate and aqueous KOH |
Answer: (C) |
| 9. | If the energies of the two photons are in the ratio of 3: 2, their wavelengths will be in the ratio of
(A) 2: 3 (B) 9: 4 (C) 3: 2 (D) 1: 2 |
Answer: (A) |
| 10. |
If the molecular wt. of Na2S2O3 and I2 are M1 and M2 respectively, then what will be the equivalent wt. of Na2S2O3 and I2 in the following reaction? 2S2 (A) M1, M2 (B) M1, M2/2 (C) 2M1, M2 (D) M1,2M2 |
Answer: (B) |
| 11. | In an alkaline medium, glycine predominantly exists as/in a /an
(A) anion (B) cation (C) covalent form (D) zwitterions |
Answer: (A) |
| 12. | In aqueous solution glucose remains as
(A) Only in open chain form (B) Only in pyranoze form (C) Only in furanose forms (D) In all three forms in equilibrium |
Answer: (D) |
| 13. | In chromite ore, the oxidation number iron and chromium are respectively ______.
(A) +3, +6 (B) +3, +2 (C) +2, +3 (D) +2, +6 |
Answer: (C) |
| 14. | In Ramsay and Rayleigh’s isolation of noble gases from air, the nitrogen of the air is finally converted into
(A) NO and NO2 (B) NaNO2 only (C) NaNO2 and NaNO3 (D) NaNO3 only |
Answer: (C) |
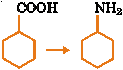
| 15. |
In the conversion
The sequence of the regents used are (A) (i) SOCl2 (ii) (B) (i) SOCl2 (ii) NH3 (C) (i) SOCl2 (ii) NH3 (iii) Heat (D) (i) SOCl2 (ii) KCN (iii) LiAlH |
Answer: (A) |