JEE MAIN, AIPMT, Chemistry Question Papers - MCQs Test 2
Quiz-summary
0 of 15 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
Information
JEE MAIN, AIPMT, Chemistry Question Papers – MCQs Test 1
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading...
You must sign in or sign up to start the quiz.
You have to finish following quiz, to start this quiz:
Results
0 of 15 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 points, (0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- Answered
- Review
-
Question 1 of 15
1. Question
1 points50 cm3 of 0.2 N HCI is titrated against 0.1 N NaOH solution. The titration is discontinued after adding 50 cm3 of NaOH. The remaining titration is completed by adding 0.5 N KOH. The volume of KOH required for completing the titration is ______.
Correct
Incorrect
-
Question 2 of 15
2. Question
1 points80 g of oxygen contains as many atoms as in
Correct
Incorrect
-
Question 3 of 15
3. Question
1 points9.65 C of electric current is passed through fused anhydrous magnesium chloride. The magnesium metal thus obtained is completely converted into a Grignard reagent. The number of moles of the Grignard reagent obtained is ______.
Correct
Incorrect
-
Question 4 of 15
4. Question
1 pointsA 6% solution of urea is isotonic with
Correct
Incorrect
-
Question 5 of 15
5. Question
1 pointsA bivalent metal has an equivalent mass of 32. The molecular mass of the metal nitrate is
Correct
Incorrect
-
Question 6 of 15
6. Question
1 pointsA body of mass 10 mg is moving with a velocity of 100 ms–1. The wavelength of de-Broglie wave associated with it would be
(Note : h = 6.63 ´ 10–34Js)
Correct
Incorrect
-
Question 7 of 15
7. Question
1 pointsA body of mass x kg is moving with a velocity of 100 ms–1. Its de Broglie wavelength is 6.62 ´ 10–35m.
Hence x is (h = 6.62 ´ 10–34Js)Correct
Incorrect
-
Question 8 of 15
8. Question
1 pointsA buffer solution contains 0.1 mole of sodium acetate dissolved in 1000 cm3 of 0.1 M acetic acid. To the above buffer solution, 0.1 mole of sodium acetate is further added and dissolved.
The pH of the resulting buffer is equal to ______.Correct
Incorrect
-
Question 9 of 15
9. Question
1 pointsA buffer solution is prepared in which the concentration of NH3 is 0.30 M and the concentration of
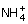 is 0.20 M. If the equilibrium constant, Kb for NH3 equals 1.8 × 10–5, what is the pH of this solution?Correct
is 0.20 M. If the equilibrium constant, Kb for NH3 equals 1.8 × 10–5, what is the pH of this solution?Correct
Incorrect
-
Question 10 of 15
10. Question
1 pointsA complex compound in which the oxidation number of a metal is zero is
Correct
Incorrect
-
Question 11 of 15
11. Question
1 pointsWhich of the following is used to prepare Cl2 gas at room temperature from concentrated HCI?
Correct
Incorrect
-
Question 12 of 15
12. Question
1 pointsWhich of the following is not an ore of magnesium?
Correct
Incorrect
-
Question 13 of 15
13. Question
1 pointsWhich of the following has the highest bond order?
Correct
Incorrect
-
Question 14 of 15
14. Question
1 pointsWhich of the following gives an aldehyde on dry distillation?
Correct
Incorrect
-
Question 15 of 15
15. Question
1 pointsWhich of the following does not give benzoic acid on hydrolysis?
Correct
Incorrect


very nice questions