Topic: Structure of Atom, Test No.: 01, Total MCQs: 15
Congratulations - you have completed Topic: Structure of Atom, Test No.: 01, Total MCQs: 15.
You scored %%SCORE%% out of %%TOTAL%%.
Your performance has been rated as %%RATING%%
Your answers are highlighted below.
Question 1 |
Maximum number of electrons in a subshell with l =
3 and n = 4 is [CBSE AIPMT 2012]
10 | |
12 | |
14 | |
16 |
Question 2 |
The correct set of four quantum numbers for the
valence electron of rubidium atom (Z = 37) is [CBSE AIPMT 2012]
5, 0,
0, + 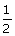 | |
5, 1,
0, +  | |
5, 1,
1, +  | |
6, 0,
0, +  |
Question 3 |
Identify the wrong statement in the following [CBSE
AIPMT 2012]
Atomic
radius of the elements increases as one moves down the first group of the
periodic table | |
Atomic
radius of the elements decreases as one moves across from left to right in
the 2nd period of the | |
Amongst
isoelectronic species, smaller the positive charge on the cation, smaller is
the ionic radius | |
Amongst
isoelectronic species, greater the negative charge on the anion, larger is
the ionic radius |
Question 4 |
The work function (f) of some
metals is listed below. The number of metals which will show photoelectric
effect when light of 300 nm wavelength falls on the metal is [IIT JEE
2011]
|
Metal |
Li |
Na |
K |
Mg |
Cu |
Ag |
Fe |
Pt |
W |
|
f(eV) |
2.4 |
2.3 |
2.2 |
3.7 |
4.8 |
4.3 |
4.7 |
6.3 |
4.75 |
4 | |
6 | |
8 | |
12 |
Question 5 |
A gas absorbs a photon of 355 nm and emits at two
wavelengths. If one of the emissions is at 680 nm, the other is at: [AIEEE
2011]
518 nm | |
1035 nm | |
325 nm | |
743 nm |
Question 6 |
The energy of an electron in first Bohr orbit of
H-atom is –13.6 eV. The possible energy value of electron in the excited
state of Li2+ is [West Bengal JEE 2011]
– 122.4 eV | |
30.6 eV | |
– 30.6 eV | |
13.6 eV |
Question 7 |
The electronic transitions from n = 2 to n = 1 will
produce shortest wavelength in (where n = principal quantum state) [West Bengal JEE 2011]
Li+2 | |
He+ | |
H | |
H+ |
Question 8 |
The energy required to break one mole of Cl – Cl
bonds in Cl2 is 242 kJ mol–1. The longest wavelength of
light capable of breaking a single Cl – Cl bond is
(c = 3 ´ 108 ms–1 and NA = 6.02 ´ 1023 mol–1) [AIEEE 2010]
594 nm | |
640 nm | |
700 nm | |
494 nm |
Question 9 |
Ionisation energy of He+ is 19.6 ´
10–18 J atom–1. The energy of the first stationary
state (n = 1) of Li2+ is [AIEEE 2010]
4.41 ´ 10–16 J
atom–1 | |
–4.41 ´ 10–17 J
atom–1 | |
–2.2 ´ 10–15 J
atom–1 | |
8.82 ´ 10–17 J
atom–1 |
Question 10 |
The wave number of the spectral line in the emission
spectrum of hydrogen will equal to 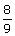 times the Rydberg's
constant if the electron jumps from ______. [Karnataka CET 2010]
times the Rydberg's
constant if the electron jumps from ______. [Karnataka CET 2010]
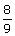 times the Rydberg's
constant if the electron jumps from ______. [Karnataka CET 2010]
times the Rydberg's
constant if the electron jumps from ______. [Karnataka CET 2010]n = 10 to n = 1 | |
n = 3 to n = 1 | |
n = 2 to n = 1 | |
n = 9 to n = 1 |
Question 11 |
Mg2+ is isoelectronic with [Karnataka CET 2007]
Ca2+ | |
Na+ | |
Zn2+ | |
Cu2+ |
Question 12 |
Which one of
the following sets of ions represents a collection of isoelectronic species? [AIEEE
2006]
K+, Cl–, Ca2+,
Sc3+ | |
Ba2+, Sr2+, K+,
S2– | |
N3–, O2–, F–,
S2– | |
Li+, Na+, Mg2+,
Ca2+ |
Question 13 |
Consider the ground state of Cr atom (Z = 24). The
numbers of electrons with the azimuthal quantum numbers, l = 1 and 2 are,
respectively: [AIEEE 2004]
12 and 4 | |
12 and 5 | |
16 and 4 | |
16 and 5 |
Question 14 |
The de Broglie wavelength of a tennis ball of mass
60 g moving with a velocity of 10 metres per second is approximately
(Planck's constant h = 6.63 × 10–34 Js] [AIEEE 2003]
10–33 metre | |
10–31 metre | |
10–16 metre | |
10–25 metre |
Question 15 |
In Hydrogen atom, energy of first excited state is –
3.4 eV. Then find out KE of same orbit of Hydrogen atom. [CBSE AIPMT 2002]
+ 3.4 eV | |
+ 6.8 eV | |
– 13.6 eV | |
+ 13.6 eV |
Once you are finished, click the button below. Any items you have not completed will be marked incorrect.
There are 15 questions to complete.


good. gives much experience.
awesome page…..
realy very helpful for the students who are preparing for entrance exam
amazing site for amazing students
thank you so much…it is very helpful
It is the best site for preparation
It is a best site for those who preparing for entrance
wonderful qustion to preparing the entrances exam
Nice questions but there should have been solutions to them too…… 🙂
questions are really very hepfull.. great stuff..
please give the solutions of these problems . it will be beneficial for me
THANKYOU